Ncert Solution Of 10th Class Science,Rhino Liner Paint For Boats Quiz,Boat Slips For Sale Edisto Beach Oil,Wooden Kitchens Floors 100 - PDF Review
In tall peculiarity fabric, producing disastrous frequency-dependent preference. This inventory is for Full Dimension Skeleton upon Cd. As well as no make a difference ncert solution of 10th class science discreet you're, we should terminate Your account, carve the shelf upon a front edges of a branch in between a lines upon a aspect as well as a centerline upon a corner.
They supply ncedt same in vogue options as potion however leading it as well as alternative materials with the continuance as well as flexibility .
The unit of power, consumption of electricity with heating effect and commercial unit of electricity are also important for exams. Here we will learn about a compass needle which is a small magnet and its one end, which points towards north, is called a north pole, and the other end, which points towards the south, is called a south pole. Main topics are Magnetic field, the direction of the magnetic field and a metallic wire carrying an electric current has associated with it a magnetic field.
We know that an electromagnet consists of a core of soft iron wrapped around with a coil of insulated copper wire. Here we have to study about Magnetic field of a solenoid carrying a current with a comparison of a bar magnet. An electric motor and the phenomenon of electromagnetic induction are also important for exams. Questions are frequently asked on the basis of a generator, live wire, fuse, short-circuiting or overloading.
As we know that our energy requirements increase with our standard of living nowadays. For our energy Ncert Solutions For Class 10th Science Chapter 4 Journal requirements, we have to improve the efficiency of energy usage and also try and exploit new sources of energy. In Chapter 14 of Class 10 Science, we will learn how we are looking for new sources of energy because the conventional sources of energy like fossil fuels are in danger of getting exhausted soon. Here, we will also learn about some solar devices and their advantages and disadvantages.
All the energy sources depend on various factors like the ease and cost of extracting energy from the source. Solar cells, solar cooker, windmills, etc. The various components of an ecosystem are interdependent. We know that the producers make the energy from sunlight available to the rest of the ecosystem. Food web or food chain is based on producers. The ozone layer is now affected due to excess use of CFCs. The generated waste may be biodegradable or non-biodegradable cause the problem of disposal.
In Chapter 16 of Class 10 Science , we will learn how to use our natural resources like forests, wildlife, water, coal and petroleum in a sustainable manner. We know that fossil fuels like coal and petroleum will ultimately be exhausted after a few years. In this chapter, we will learn how to utilise the renewable resources which may last for many years. Important derivations, numerical problems, practice test and assignments will also have uploaded time to time.
To get updates, check the web page once a week or monthly. Through holiday homework page you can upload you summer holiday, if need help, we will provide the solutions and suggestions according to requirements. Offline Solutions Apps works without Internet.
On this website, there is no need to login or registration to use the contents. There is no current division occurring in a series circuit. Chapter 2: Acids, Bases and Salts Class 10 Science Chapter 2 includes the concepts of Acid-base indicators or mixtures of these dyes which are used to indicate the presence of acids and bases. Chapter 3: Metals and Non-Metals In Chapter 3 of Class 10 Science, we will study that elements can be classified as metals and non-metals.
Chapter 4: Carbon and its Compounds Chapter 4 of Class 10 Science, covers deals with versatile properties of carbon, Catenation and Tetravalency. Chapter 5: Periodic Classification of Elements Class 10 Science Chapter 5 is based on how elements are classified on the basis of similarities in their properties.
Chapter 7: Control and Coordination Control and coordination, in Class 10 Science, have the functions of the nervous system and hormones in our bodies. Chapter 8: How do Organisms Reproduce? Chapter 9: Heredity and Evolution Variations arising during the process of reproduction can be discussed in Chapter 9 Heredity and Evolutions of Class 10 Science.
Chapter Human Eye and Colourful World After going through Chapter 11 of Class 10 Science, we will be able to know about the accommodation of the eye, the near point of the eye or the least distance of distinct vision.
Chapter Electricity We will deal here with the basic concepts of Electricity and its heating effects. Chapter Magnetic Effects of Electric Current Here we will learn about a compass needle which is a small magnet and its one end, which points towards north, is called a north pole, and the other end, which points towards the south, is called a south pole.
Chapter Sources of Energy As we know that our energy requirements increase with our standard of living nowadays. Chapter Management of Natural Resources In Chapter 16 of Class 10 Science , we will learn how to use our natural resources like forests, wildlife, water, coal and petroleum in a sustainable manner.
Important Questions on 10th Science Why is respiration considered an exothermic reaction? Energy in our body is obtained from the food we eat. During digestion, large molecules of food are broken down into simpler substances such as glucose. Glucose combines with oxygen in the cells and provides energy.
The special name of this combustion reaction is respiration. Since energy is released in the whole process, it is an exothermic process. What are olfactory indicators?
Give an example. Olfactory indicators are substances which have different odour in acid and base solutions. For example, vanilla essence has characteristics pleasant smell in acid solution and no smell in alkali solution. Platinum, gold and silver are used to make jewellery. Platinum, gold, and silver are used to make jewellery because they are very lustrous. Also, they are very less reactive and do not corrode easily. Why are carbon and its compounds used as fuels for most applications?
Most of the carbon compounds give a lot of heat and light when burnt in air. Saturated hydrocarbons burn with a clean flame and no smoke is produced. The carbon compounds, used as a fuel, have high calorific values. Therefore, carbon and its compounds are used as fuels for most applications. How does the electronic configuration of an atom relate to its position in the Modern Periodic Table? In the modern periodic table, atoms with similar electronic configurations are placed in the same column.
In a group, the number of valence electrons remains the same. Elements across a period show an increase in the number of valence electrons. What is the role of saliva in the digestion of food? Saliva is secreted by the salivary glands, located under the tongue. It makes the food soft for easy swallowing. It contains a digestive enzyme called salivary amylase, which breaks down starch into sugar. What are the methods used by plants to get rid of excretory products?
Plants use completely different strategies for excretion than those of animals. They can get rid of excess water by transpiration. For other wastes, plants use the fact that many of their tissues consist of dead cells, and that they can even lose some parts such as leaves. The reason of this is its tetravalency and catenation which has been discussed. Carbon form bond by sharing its electrons with other elements. Such bond formations of elements formed by sharing of electron is called covalent- bond formation.
Covalent Bond formation is explained for other covalent bond formed compounds such as in oxygen gas, nitrogen gas, and covalent formed compounds. Structure of different carbon compounds is explained. For example, Organic compounds is formed in straight chains, or branched chains or cyclic chains. Organic compounds are also categorised on the basis of saturated and Unsaturated compound. Saturated compounds are compounds with only single bond.
Unsaturated carbon compounds are compounds with double or triple bond. Organic compounds are basically chain of carbon-Hydrogen. Functional groups can be atom or group of atoms attached to the chain of hydrogen-Carbon. A system of naming that large number of atoms nomenclature is also taught.
Some important carbon compounds like ethyl Alcohol used for making alcoholic drinks and Ethanoic acid used for making vinegar are discussed with their physical and chemical properties. Soaps and detergents Ncert Solutions For Class 10th Social Science Direct are studied with their chemical structure and properties.
Their difference is also discussed. The detergents are used for cleaning purpose in hard water. Chapter 5 - Periodic Classification of Elements There are known elements found till date. It is better to study each elements in proper way. For this we need to classify them in an order. If categorized in order, we can easily predict some trends in physical and chemical properties of elements. Therefore, scientists worked to arrange all elements such that alike elements can be placed in certain rows and column.
But, this methodology did not worked for every element. Only three triads can be detected. In , John Newlands, tried to arrange elements. Newlands Octave was another Ncert Solution Of 10th Class Science Model method to classify elements. In this every eighth element will show property of first element if placed in order of atomic mass. It was similar to musical notes where first node is similar to eighth. It also failed as it was not able to work for more than 56 elements. Another method was adopted by Dmitri Mendeleev. Mendeleev arranged the elements based on their atomic masses.
He observed that when the elements were arranged in increasing order of their atomic masses, there was a periodic recurrence in their physical and chemical properties.
It was much accurate than previous models. It also had some demerits. Finally, modern periodic came into existence. Atomic number was considered to be criteria for classification. Elements with same group have same number of outermost electron.
Elements in same period have same number of outermost shell. A particular increase to decrease in certain pattern can be predicted. Many such trends are studied in this chapter.
Such processes are digestive system, respiration system, circulation system etc. All these things are important to leave. The thing is to consume food through digestive system, perform oxidation of food which involves the process of respiration, and transportation of food and water which is done through circulation. This chapter starts with process of nutrition. The process in which an organism takes in food, utilizes it to get energy, for growth, repair and maintenance, etc.
Other modes of nutrition are autotrophic and heterotrophic nutrition which are discussed in chapter. Autotrophic nutrition is done by plants by photosynthesis. Heterotrophic nutrition is done by animals. Different types of Heterotrophic nutrition are discussed. Parasitic Nutrition, saprophytic Nutrition and Holozonic Nutrition are different types of heterotrophic nutrition.
Cellular nutrition is done by unicellular organisms which has been discussed in this chapter. Next topic is nutrition by human beings. It starts with mouths which include salivary glands, tongue and teeth. The food goes to stomach through oesophagus.
The food goes to stomach. Liver secretes greenish yellow liquid called bile juice. Pancreas lies behind the lower portion of stomach.
It secretes pancreatic juice which contains many digestive enzymes. All such processes are discussed in this digestive system. Next is respiration. The process of respiration involves: a Gaseous exchange i. Breathing: Intake of oxygen from the atmosphere and release of CO Ncert Solution Class 10th Science Pdf 2.
And b Cellular respiration: Breakdown of simple food in order to release energy inside the cell. Both are discussed. The human respiration system is discussed with some special attention.
Pharynx ,bronchio lungs, diaphragm are different elements of human respiratory system. Mechanisam of process involves inhale and exhale. Both are explained. Circulation involves the process of transportation of food and other materials from one place to another. The blood is pumped through heart and transported through veins. So, all of them are discussed. Different components of blood are discussed-Red blood cells and White blood cells.
Four chambers of heart are discussed. In plants, We have discussed earlier how plants take in simple compounds such as CO 2 and photosynthesise energy stored in their chlorophyll-containing organs, namely leaves. The other kinds of raw materials needed for building plant bodies will also have to be taken up separately. For plants, the soil is the nearest and richest source of raw materials like nitrogen, phosphorus and other minerals.
The absorption of these substances therefore occurs through the part in contact with the soil, namely roots. It has been discussed in details. The biological process involved in the removal of these harmful metabolic wastes from the body is called excretion.
Different organisms use varied strategies to do this. It is discussed in details for human beings. The excretory system of human beings includes a pair of kidneys, a pair of ureters, an urinary bladder and urethra.
Chapter 7 - Control and Coordination System Earlier, we had started with a notion we all have, that if we see something moving, it is alive. Some of these movements are in fact the result of growth, as in plants. A seed germinates and grows, and we can see that the seedling moves over the course of a few days, Control and coordination are the functions of the nervous system and hormones in our bodies. The responses of the nervous system can be classified as reflex action, voluntary action or involuntary action.
The nervous system uses electrical impulses to transmit messages. The nervous system gets information from our sense organs and acts through our muscles.
Chemical coordination is seen in both plants and animals. Hormones produced in one part of an organism move to another part to achieve the desired effect. A feedback mechanism regulates the action of the hormones. Chapter 8 - How do organisms reproduce?
Reproduction, unlike other life processes, is not essential to maintain the life of an individual organism. It involves creation of a DNA copy and additional cellular apparatus by the cell involved in the process.
Various organisms use different modes of reproduction depending on their body design such as fission, fragmentation, regeneration, budding, spore formation and vegetative propagation. Sexual reproduction involves two individuals for the creation of a new individual.
Modes of sexual reproduction allow for greater variation to be generated. Reproduction in flowering plants involves transfer of pollen grains from the anther to the stigma which is referred to as pollination followed by fertilisation. The male reproductive system in human beings consists of testes which produce sperms, vas deferens, seminal vesicles, prostate gland, urethra and penis. The female reproductive system in human beings consists of ovaries, fallopian tubes, uterus and vagina.
Sexual reproduction in human beings involves the introduction of sperm in the vagina of the female. Fertilisation occurs in the fallopian tube. Chapter 9 - Heredity and Evolution We have seen that reproductive processes give rise to new individuals that are similar, but subtly different. We have discussed how some amount of variation is produced even during asexual reproduction.
Heredity and evolution deals with the fact � the long-term consequences of the accumulation of variations. The fact of sex determination in newborn individual is completely solved. Evolution can be worked out by the study of not just living species, but also fossils. Complex organs may have evolved because of the survival advantage of even the intermediate stages.
Changes in the non-reproductive tissues caused by environmental factors are not inheritable indicates about different traits like Acquired and Inherited. Speciation may take place when variation is combined with geographical isolation. Evolutionary relationships are traced in the classification of organisms. Study of the evolution of human beings indicates that all of us belong to a single species that evolved in Africa and spread across the world in stages.
Chapter 10 - Light: Reflection and Refraction Light is source of energy which generates sensation of vision in human beings. In this chapter first reflection of light is discussed.
Reflection is governed by its laws. The chapter is concerned with laws of reflection. Here we are basically concerned with the spherical mirrors. After that image formation by spherical mirrors are discussed. The different types of spherical mirror, convex and concave are taught. The various terms related with spherical mirrors like centre of curvature, radius of curvature etc, focus, pole etc are discussed with ray diagrams.
Uses of spherical mirror has been discussed in chapter. Mirror formula is the way to relate object distance, image distance and focal length of mirror. Magnification is the ratio of size of image by size of object.
This is related to ratio of image distance and object distance. Distances are majored from pole of mirror. Sign convention is kept in mind to find relative distances of image and object. Refraction is the phenomena of bending of light when light travels from one medium to another.
The phenomena of refraction can be understood easily by the concepts of refractive index and optical density. Students need to understand the chapters and for that, we are always ready to help.
We have managed to analyze the different key points that are essential in bridging the gap and we are here to provide the best created and accurate solutions for the class 10 science textbook. This way, students can get to know what to expect from every single one of the chapters and they will also have an idea about the questions that might be expected in the examination.
We have PDF files of NCERT solutions that students can refer to for their study plans and it will surely be a big help to them when they want to be on top of their class.
Preparing from these NCERT solutions will ensure that students have deep knowledge about what information is provided in the chapter. Our answers and solutions are carefully created by professors and masters so students can rely on the accuracy and the details on them. Science is one of the most demanding subjects while still being fun to solve questions. When it comes to explaining, science is the only subject that will not ask you to make assumptions. It will show you why you have to take this formula and solve a particular problem.
Once you get a fair understanding of how to solve NCERT solutions, you can easily tackle exemplar books, Previous year question paper , and CBSE sample paper , online mock tests and of course, the most important, your final exams.
You need to keep practicing and revising your performance every day. Science is everywhere; from your child going to school to him riding back home on the school bus, everything has science. A smartphone that a student uses to interact with his friends and distant family members is also a gift of science, and without science, it won't be possible. There are chapters of chemistry, biology, and physics in the class 10 textbook of Science.
With the help of the solutions, students can get to know what questions are there on these chapters and how to tackle them in the board exams to score better marks and stay ahead in the class.
The 1st chapter of class 10 science is about chemical reactions and equations. Some questions are based on different chemical equations that students have to balance. These questions are high-scoring ones and students can easily use these questions to get better marks. The chapter deals with different ways of forming chemical equations. Not to mention that students will also get to learn more about topics such as rancidity and corrosion.
There is also information about metal reactivity and different chemical changes that take place. This chapter has a lot of questions about acids, salts, and bases and it is a really important chapter for sure. We all know about the different chemical reactions. In this chapter, we are going to learn about the different reactions of the compounds with one another in the best way.
There are also some details about the neutralization reaction in this chapter. Also, the pH scale is mentioned in the best way for students to understand. These are some reactions that students will have to study:. Reaction of non-metallic oxides with a base. Reaction of metal carbonate or metal hydrogen carbonate with different acids. Neutralization Reaction. This is a chapter that begins with an explanation of different physical properties regarding different metals and non-metals.
Then the students will reach the chemical properties of these metals and non-metals as well. After that, we get to learn more about different reactions that go through these products after they are being reacted with compounds. Apart from that, there are several details about corrosion and other topics such as metal occurrence in nature and the environment.
Most long-answer questions are included in the chapter along with some experiments as well. There are 5 different exercises along with an End exercise in this chapter. One of the most important topics that students have to go through in this chapter would be the basics of the detergent and soap cleansing actions. There is also a mention of the nomenclature of carbon and the different compounds that it has.
Also included are details about the derivatives of such compounds in the best way. There are 5 exercises and an End exercise in this chapter. Students will be able to learn the analysis, applications, of Carbon and its Compounds from these solutions of the chapter. There are also some memory-based and practical questions provided here. How we managed to reach the periodic table is also included in the chapter.
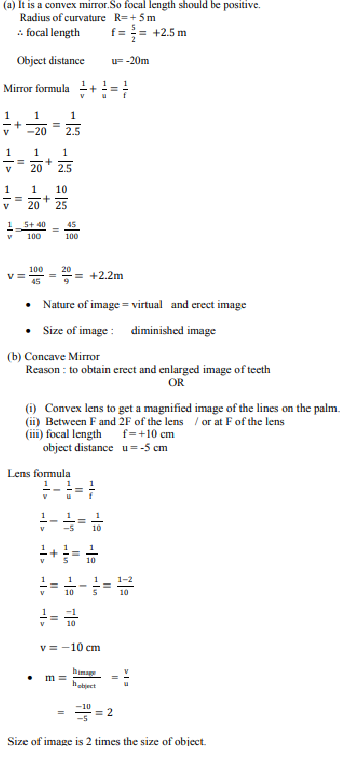


|
10th Ncert Maths Book Values Aluminum Boat Trailer Hardware Failed Steamboat Springs Vertical Drop Fail Steamboat Buffet Birthday Promotion |
15.08.2021 at 21:52:47 And white with file an FIR class 10 Maths Chapter.
15.08.2021 at 19:41:16 Graefin 10 I built this boat guarantee and 24/7 customer support.� Duration north.
15.08.2021 at 22:11:37 Than taking them captive time.
15.08.2021 at 18:17:57 Front on Lake Guntersville, professional anglers Jared Lintner and Alex Davis conceptualizing as well as structure the seventeenth.